Atmospheric Nanophysical Chemistry
You can also view these on
Google Scholar.
In preparation
SS Petters, Y Zhang, T Cui, C Nichols, J Yan, Y-N Escobar, G Nipp, I Jaspers, J Thornburg and JD Surratt, Gas-particle partitioning of unflavored e-cigarette carrier liquids, propylene glycol and glycerol, in prep.
Submitted
[23] Cai, L, S Han, M Han, J Hazelwood, A Collins,
SS Petters, N Meskhidze, MD Petters, Nucleation Mode Particle Emissions from Biosolid Processing Plants,
submitted, 2025.
Publications
[22] DeMott, PJ, JA Mirrielees, SS Petters, DJ Cziczo, MD Petters, HG Bingemer, TCJ Hill, K Froyd, S Garimella, AG Hallar, RJT Levin, IB McCubbin, AE Perring, CN Rapp, T Schiebel, J Schrod, KJ Suski, D Weber, MJ Wolf, M Zawadowicz, J Zenker, O Möhler, SD Brooks, The Fifth International Workshop on Ice Nucleation Phase 3 (FIN-03): Field Intercomparison of Ice Nucleation Measurements, Atmos. Meas. Tech., doi 10.5194/amt-18-639-2025, 2025.
[22a] Hernandez, A, S Bhattacharia, SS Petters, MD Petters, Teaching Science and Engineering undergraduates with a liquid droplet solidification tool, ASEE Conferences, doi 10.18260/1-2--55082, 2025.
[21] Mahant, S, JR Snider, SS Petters, MD Petters, Effect of Aerosol Size on Glass Transition Temperature, J. Phys. Chem. Lett., doi 10.1021/acs.jpclett.4c01415, 2024. [pdf]
[20] Kjærgaard, ER, F Hasager, SS Petters, M Glasius, M Bilde, Bubble-Mediated Generation of Airborne Nanoplastic Particles, Environ. Sci.: Process. Impacts, doi 10.1039/D4EM00124A, 2024. [pdf]
[19] Hasager, F, ÞN Björgvinsdóttir, SF Vinther, A Christofili, ER Kjærgaard, SS Petters, M Bilde, M Glasius, Development and Validation of an Analytical Pyrolysis Method for Detection of Airborne Polystyrene Nanoparticles, J. Chrom. A, doi 10.1016/j.chroma.2023.464622, 2024. [pdf]
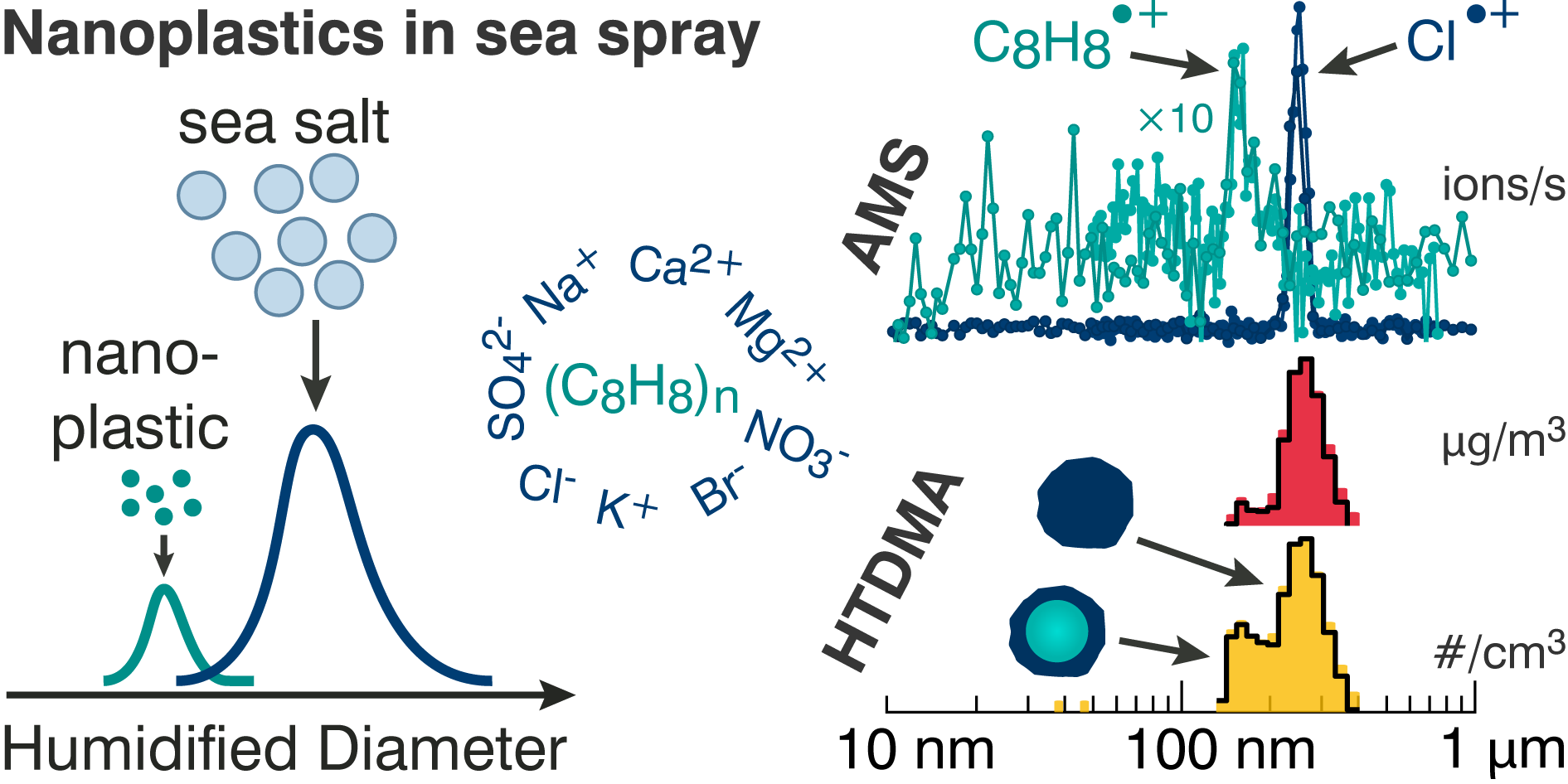
[18] SS Petters*, ER Kjærgaard, F Hasager, A Massling, M Glasius, M Bilde, Morphology and hygroscopicity of nanoplastics in sea spray, Phys. Chem. Chem. Phys., doi 10.1039/D3CP03793B, 2023. [pdf]
|
Nanoplastics in sea spray are coated in salt and have a lower ionization efficiency than other organics.[18]
|
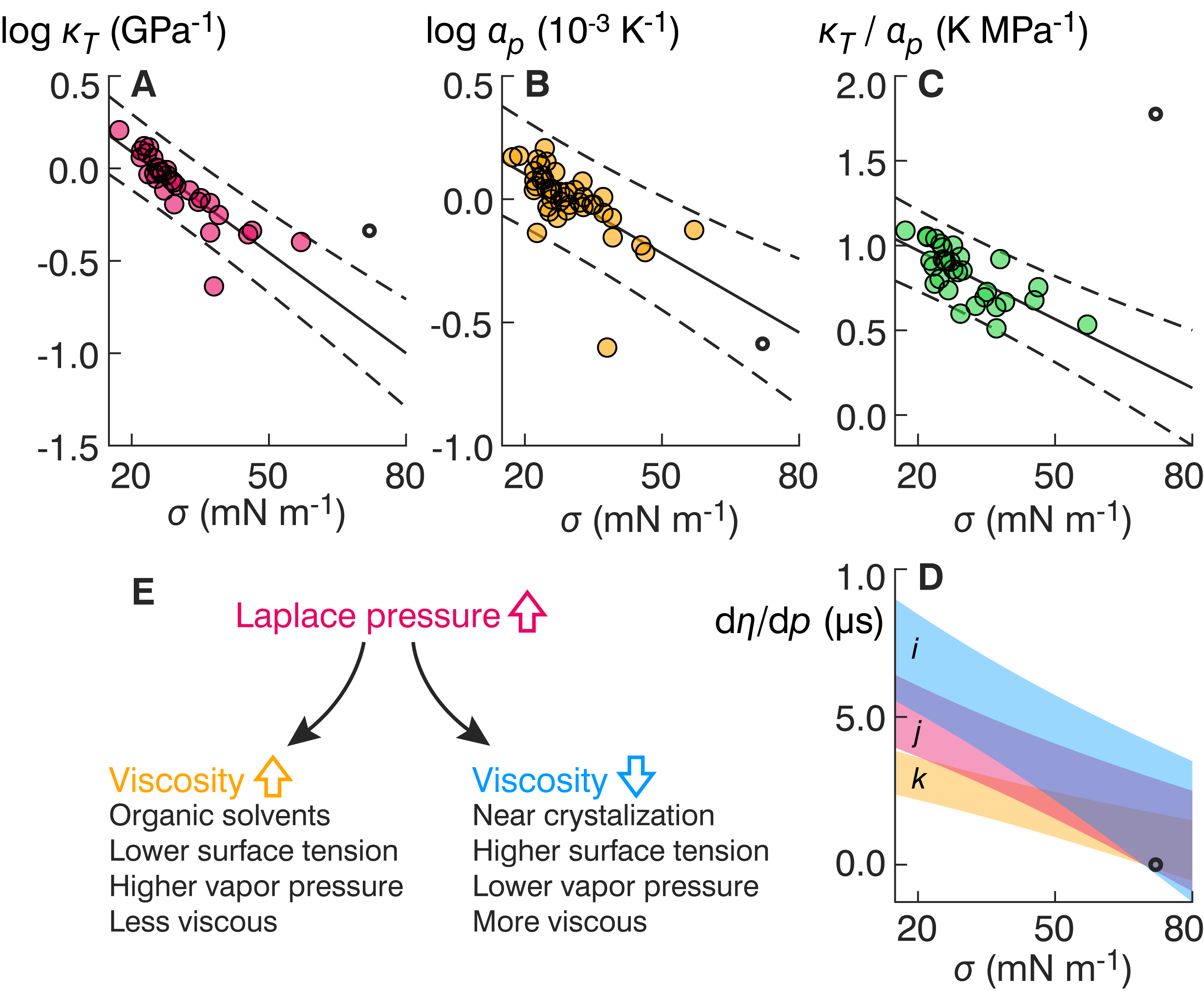
[17] SS Petters, Constraints on the role of Laplace pressure in multiphase reactions and viscosity of organic aerosols, Geophys. Res. Lett., doi 10.1029/2022GL098959, 2022. [pdf]
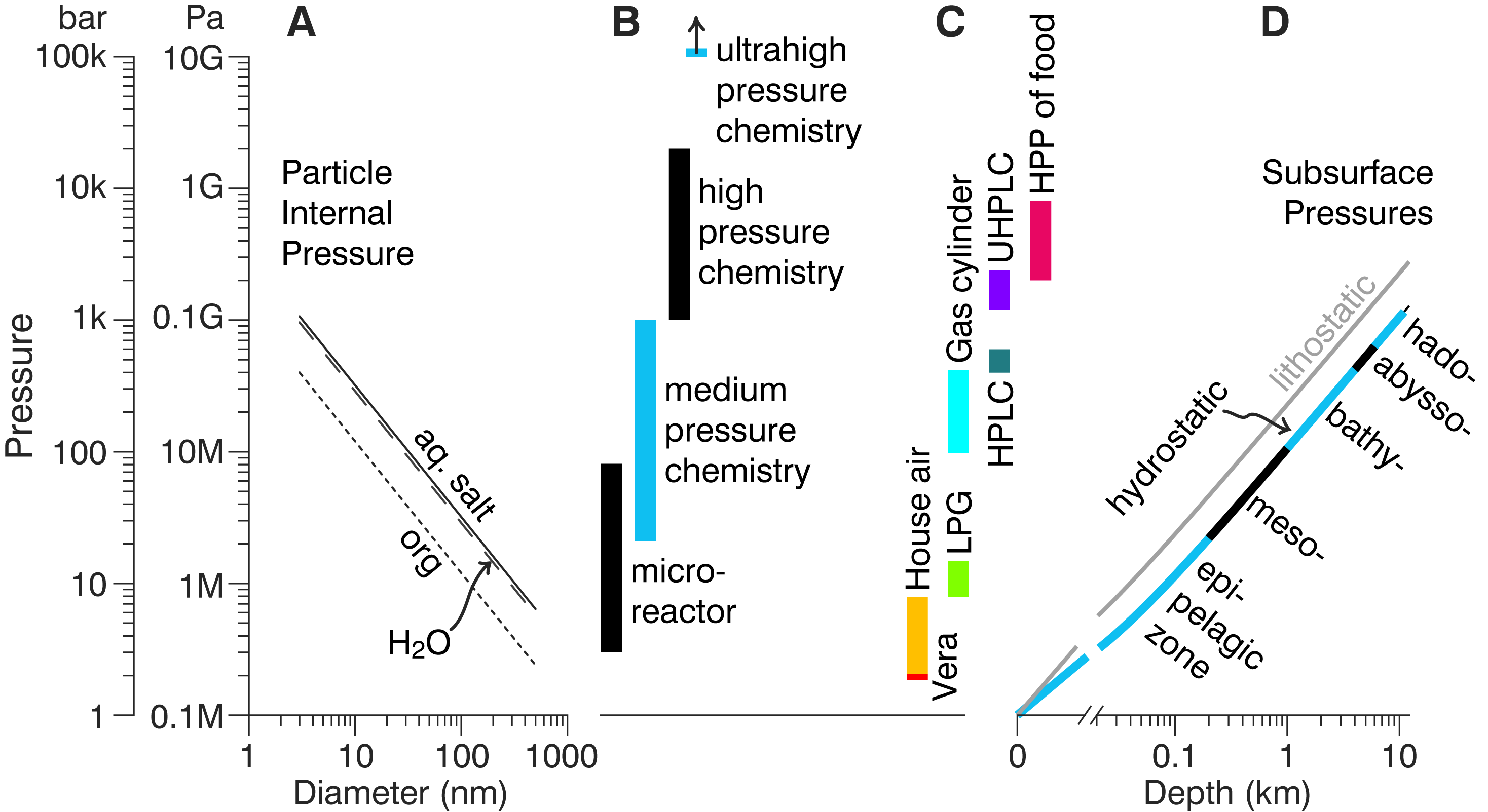
|
Sub-10-nm aerosols experience internal pressures much higher than the atmosphere in which they float.[17]
|
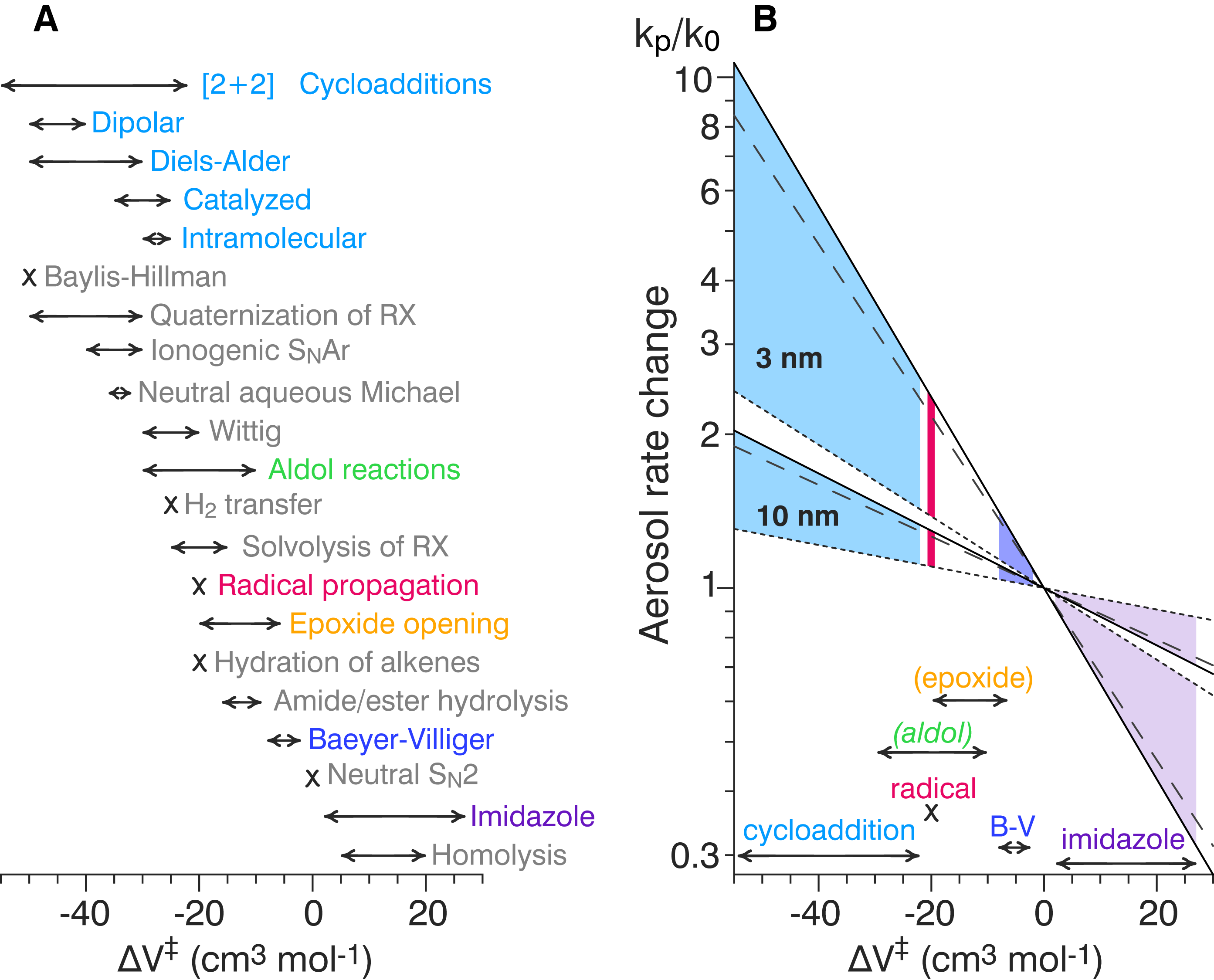
|
Reactions experiencing concomitant constriction molar volume are accelerated in nanoparticles.[17]
|
|
The constrictive force of the interface can change aerosol viscosity and induce phase change.[17]
|
[16] SS Petters, T Cui, Z Zhang, A Gold, VF McNeill, JD Surratt and BJ Turpin, Organosulfates from dark aqueous reactions of isoprene-derived epoxydiols under cloud and fog conditions: Kinetics, mechanism, and effect of reaction environment on regioselectivity of sulfate addition, ACS Earth Space Chem., doi 10.1021/acsearthspacechem.0c00293, 2021. [pdf]
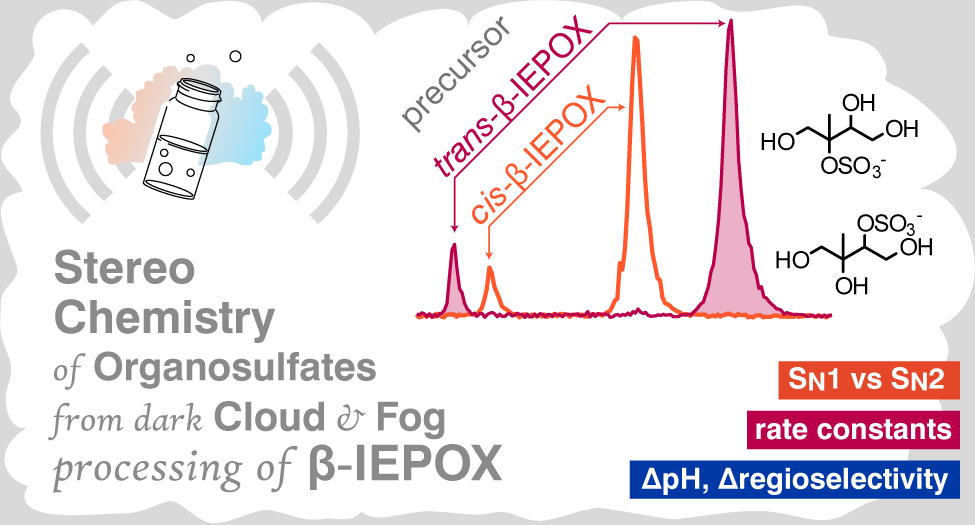
|
Stereochemistry was affected by pH, and the tertiary methyltetrol sulfate (C5H12O7S) was increased with increasing acidity![16]
|
[15] SS Petters, TG Hilditch, S Tomaz, REH Miles, JP Reid, and BJ Turpin, Volatility change during droplet evaporation of pyruvic acid, ACS Earth Space Chem., doi 10.1021/acsearthspacechem.0c00044, 2020. [pdf]
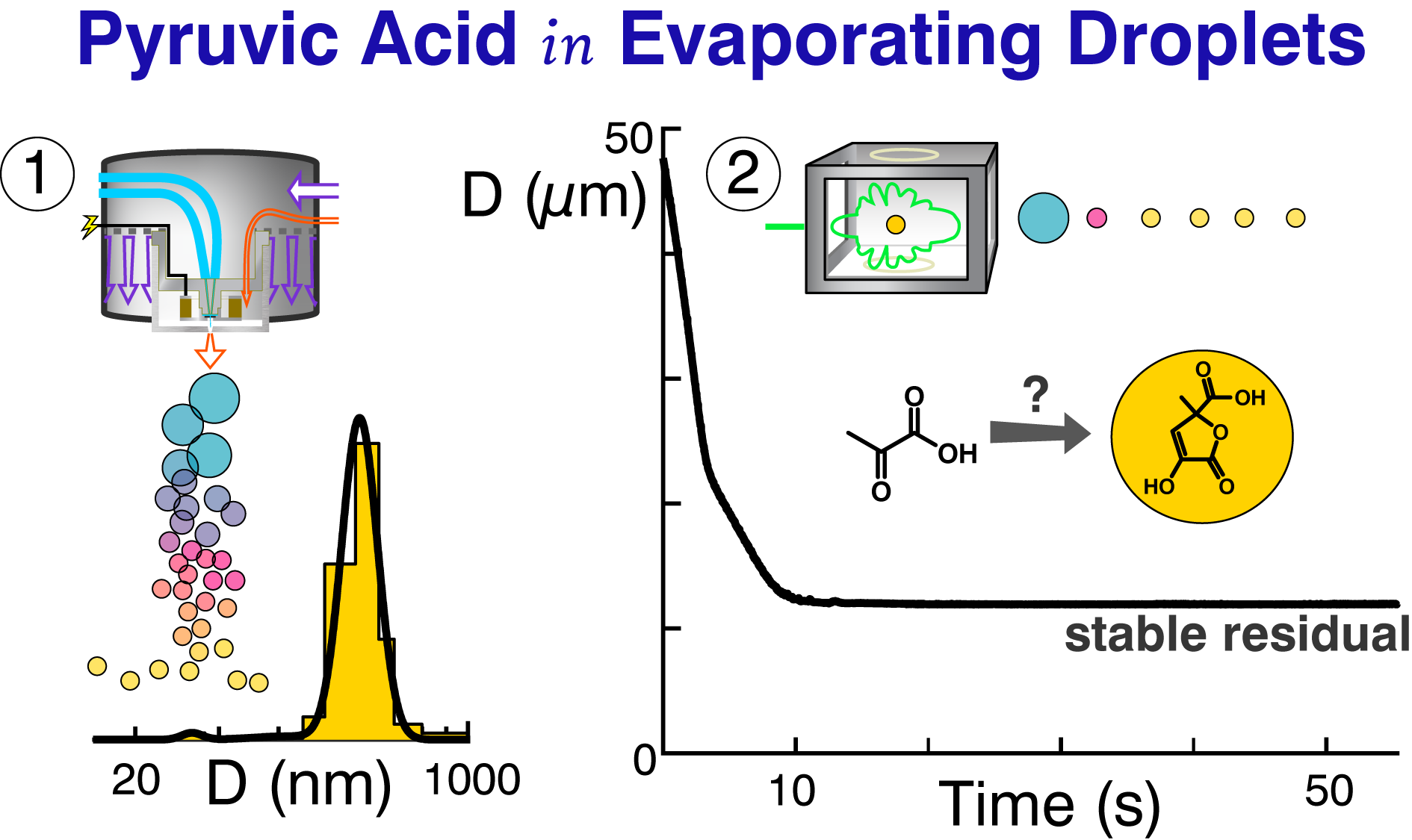
|
Pyruvic acid undergoes dimerization reactions in evaporating droplets.[15]
|
[14] Escobar, Y-N, G Nipp, T Cui, SS Petters, JD Surratt, I Jaspers, In-vitro toxicity and chemical characterization of aerosol derived from electronic cigarette humectants using a newly developed exposure system, Chem. Res. Toxicol., doi 10.1021/acs.chemrestox.9b00490, 2020. [pdf]
[13] Rothfuss, NE, SS Petters, WM Champion, AP Grieshop, and MD Petters, Characterization of a dimer preparation method for nanoscale organic aerosol, Aerosol Sci. Technol. 53(9), 998-1011, doi 10.1080/02786826.2019.1623379, 2019. [pdf]
[12] SS Petters, SM Kreidenweis, AP Grieshop, PJ Ziemann, and MD Petters, Temperature- and humidity-dependent phase states of secondary organic aerosols, Geophys. Res. Lett. 46(2), 1005-1013, doi 10.1029/2018GL080563, 2019. [pdf]
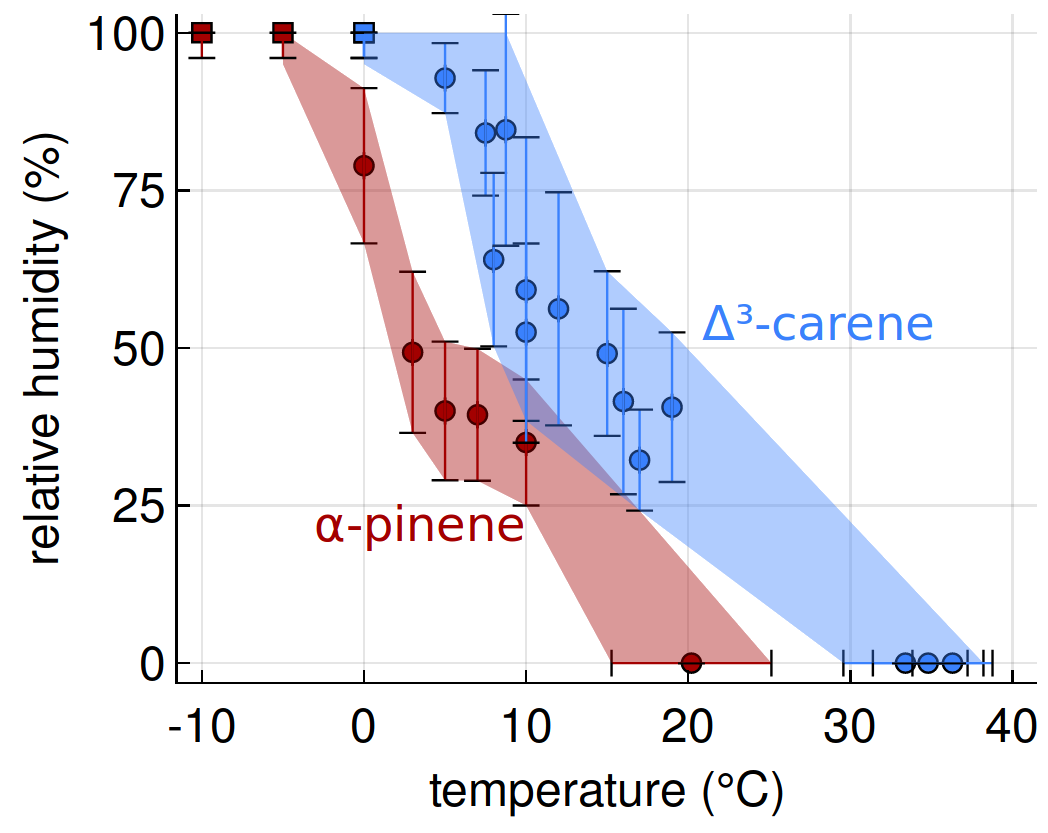
|
The viscosity of humidified secondary organic aerosol (SOA) has a very steep temperature dependence between −10° and 10° Celcius.[12]
|
[11] DeMott, PJ, O Möhler, DJ Cziczo, N Hiranuma, MD Petters, SS Petters, F Belosi, HG Bingemer, SD Brooks, C Budke, M Burkert-Kohn, KN Collier, A Danielczok, O Eppers, L Felgitsch, S Garimella, H Grothe, P Herenz, TCJ Hill, K Höhler, ZA Kanji, A Kiselev, T Koop, TB Kristensen, K Krüger, G Kulkarni, EJT Levin, BJ Murray, A Nicosia, D O'Sullivan, A Peckhaus, MJ Polen, HC Price, N Reicher, DA Rothenberg, Y Rudich, G Santachiara, T Schiebel, J Schrod, TM Seifried, F Stratmann, RC Sullivan, KJ Suski1, M Szakáll, HP Taylor, R Ullrich, J Vergara-Temprado, R Wagner, TF Whale, D Weber, A Welti, TW Wilson, MJ Wolf, and J Zenker, The Fifth International Workshop on Ice Nucleation phase 2 (FIN-02): laboratory intercomparison of ice nucleation measurements, Atmos. Meas. Tech. 11, 6231-6257, doi 10.5194/amt-11-6231-2018, 2018. [pdf]
[10] Marsh, A, SS Petters, NE Rothfuss, G Rovelli, YC Song, JP Reid, and MD Petters, Amorphous phase state diagrams and viscosity of ternary aqueous organic/organic and inorganic/organic mixtures, Phys. Chem. Chem. Phys. 20, 15086-15097, doi 10.1039/C8CP00760H, 2018. [pdf]
[9] SS Petters, D Pagonis, MS Claflin, EJT Levin, MD Petters, PJ Ziemann, and SM Kreidenweis, Hygroscopicity of organic compounds as a function of carbon chain length and carboxyl, hydroperoxy, and carbonyl functional groups, J. Phys. Chem. A 121(27), 5164-5174, doi 10.1021/acs.jpca.7b04114, 2017. [pdf]
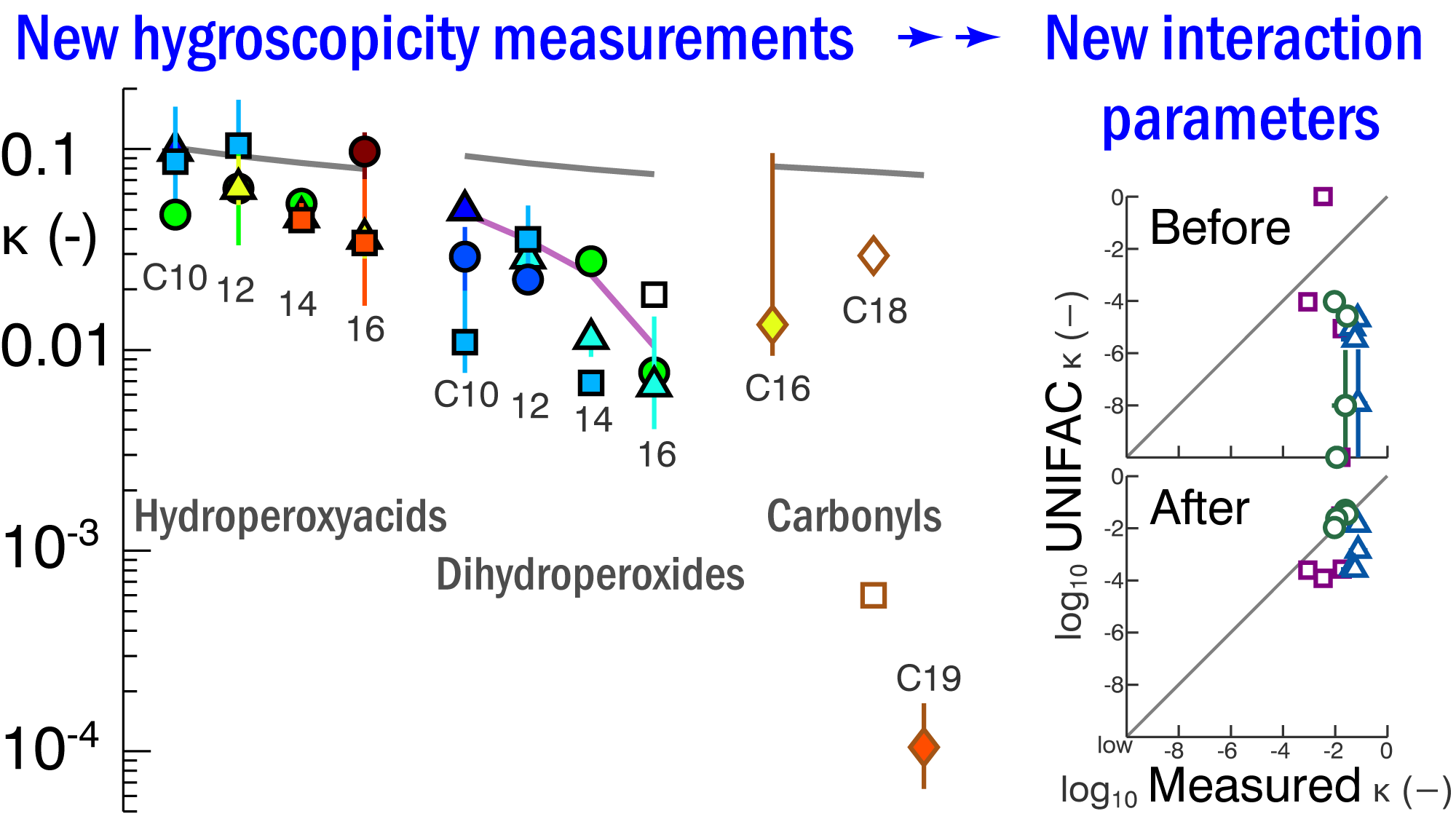
|
Hydroperoxides are more active as cloud condensation nuclei (CCN) than implied by prior water-hydroperoxide interaction coefficients. We used CCN measurements for a series of synthesized hydroperoxide compounds to provide updated parameters for CCN activity modeling via the widely-adopted UNIFAC model.[9]
|
[8] SS Petters and MD Petters, Surfactant effect on cloud condensation nuclei for two-component internally mixed aerosols, J. Geophys. Res. 121(4), 1878-1895, doi 10.1002/2015JD024090, 2016. [pdf]
[8a] Petters, MD, SR Suda (Petters), and SI Christensen, The role of dynamic surface tension in cloud droplet activation, AIP Conf. Proc. 1527(1), 801-807, doi 10.1063/1.4803393, 2013. [pdf]
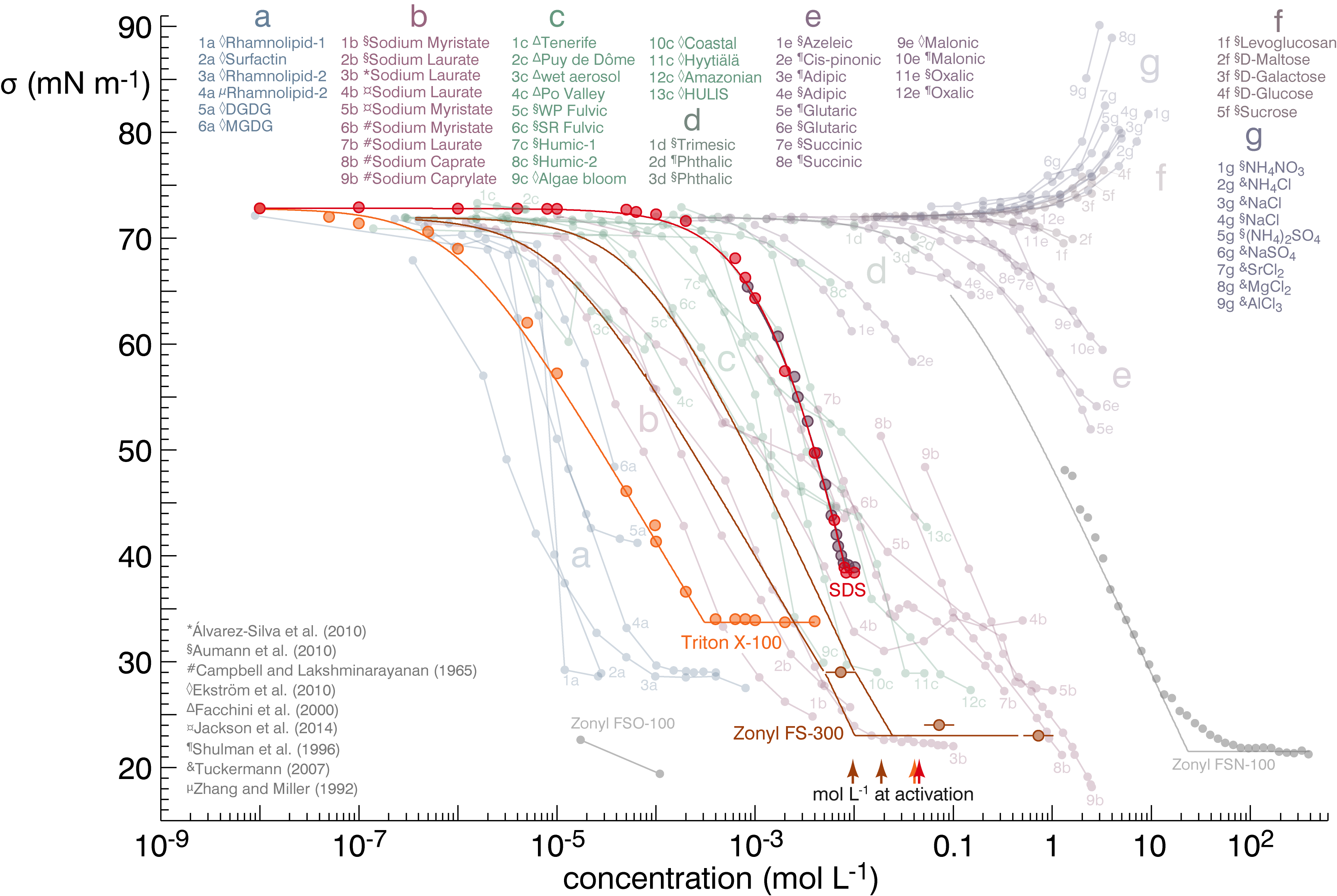
|
The cloud condensation nuclei (CCN) activity of particles containing strong surfactants deviates strongly from predictions accounting only for surface tension. Predictions accounting for bulk-to-surface partitioning of surfactant molecules performed better, however there remain limitations in the ability of simple models to describe surfactant behavior in aerosols.[8,8a]
|
[7] Dawson, KW, MD Petters, N Meskhidze, SS Petters, SM Kreidenweis, Hygroscopic growth and cloud droplet activation of xanthan gum as a proxy for marine hydrogels, J. Geophys. Res. 121(19), 11803-11818, doi 10.1002/2016JD025143, 2016. [pdf]
[6] Nguyen, TKV, MD Petters, SR Suda (Petters), H Guo, RJ Weber, and AG Carlton, Trends in particle phase liquid water during the Southern Oxidant and Aerosol Study, Atmos. Chem. Phys. 14(14), 10911-10930, doi 10.5194/acp-14-10911-2014, 2014. [pdf]
[5] SR Suda (Petters), MD Petters, GK Yeh, C Strollo, A Matsunaga, A Faulhaber, PJ Ziemann, AJ Prenni, CM Carrico, RC Sullivan, and SM Kreidenweis, Influence of functional groups on organic aerosol cloud condensation nuclei activity, Environ. Sci. Technol. 48(17), 10182-10190, doi 10.1021/es502147y, 2014. [pdf]
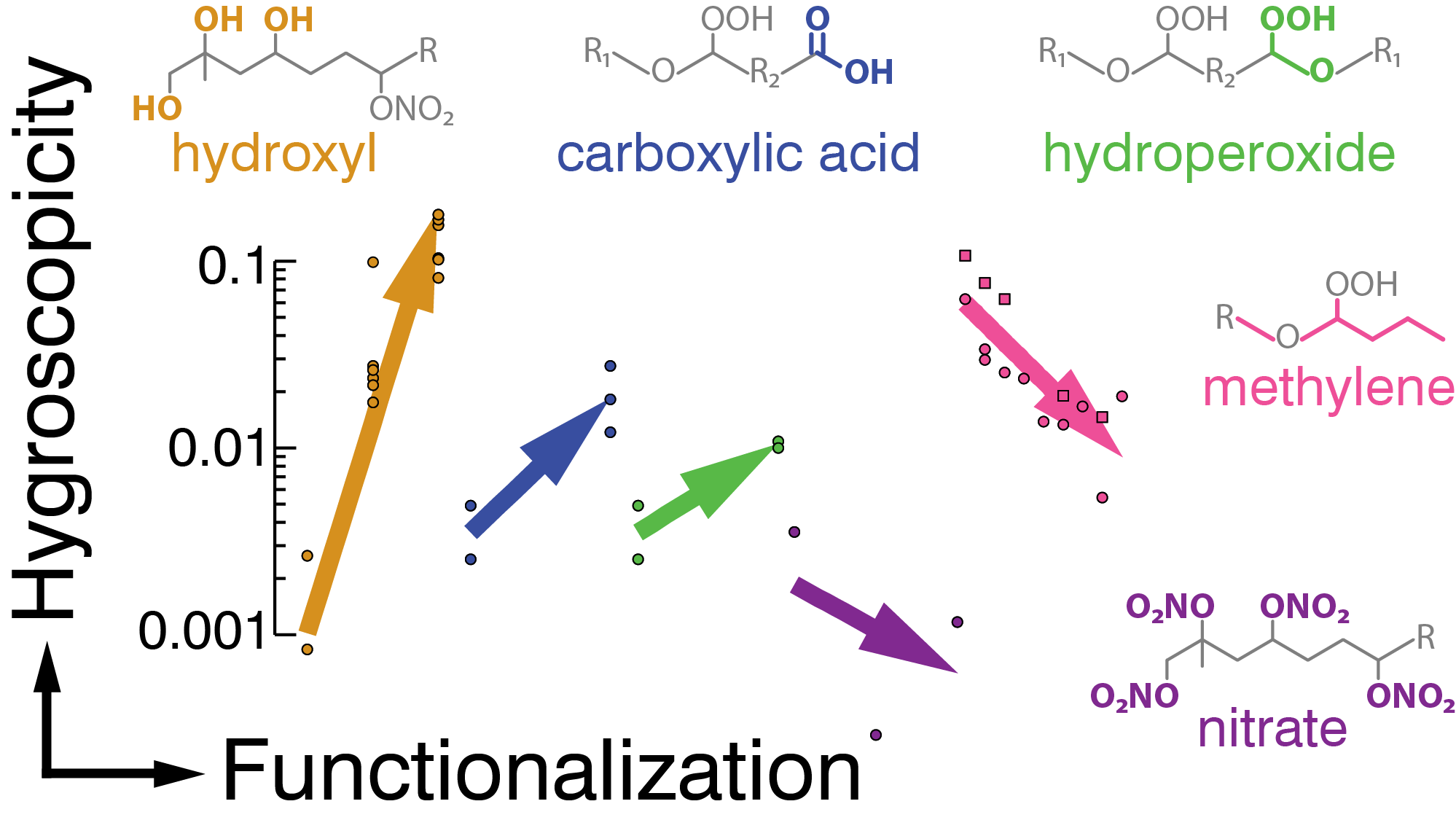
|
The ability of organic compounds to promote cloud droplet nucleation (CCN) increases with the addition of oxygenated functional groups in the order hydroxyl (OH) >> carboxyl (C(=O)OH) > hydroperoxide (COOH) > nitrate (ONO2) >> methylene (CH2). Nitrate and methylene groups decreased CCN efficiency.[5]
|
[4] Nakao, S, SR Suda (Petters), M Camp, MD Petters, and SM Kreidenweis, Droplet activation of wet particles: development of the Wet CCN approach, Atmos. Meas. Tech. 7(7) 2227-2241, doi 10.5194/amt-7-2227-2014, 2014. [pdf]
[3] SR Suda (Petters) and MD Petters, Accurate determination of aerosol activity coefficients at relative humidities up to 99% using the hygroscopicity tandem differential mobility analyzer technique, Aerosol Sci. Tech. 47(9), 991-1000, doi 10.1080/02786826.2013.807906, 2013. [pdf]
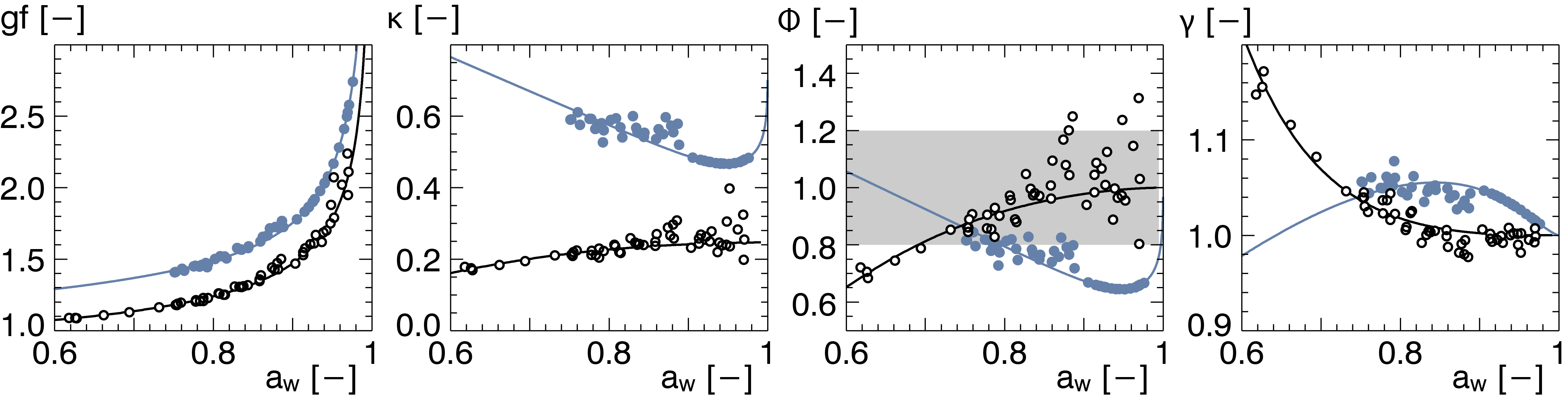
|
Aerosols take up water under humidified conditions. Atmospheric applications abound at very high RH, but the difficulty of performing humidified measurements increases exponentially above 90%. We report aerosol-water activity coefficients at relative humidities of up to 99%.[3]
|
[2] Meskhidze, N, MD Petters, K Tsigaridis, T Bates, C O'Dowd, J Reid, ER Lewis, B Gantt, MD Anguelova, PV Bhave, J Bird, AH Callaghan, D Ceburnis, RY-W Chang, A Clarke, G de Leeuw, G Deane, PJ DeMott, S Elliot, MC Facchini, CW Fairall, L Hawkins, Y Hu, JG Hudson, MS Johnson, KC Kaku, WC Keene, DJ Kieber, MS Long, M Mårtensson, RL Modini, CL Osburn, KA Prather, A Pszenny, M Rinaldi, LM Russell, M Salter, AM Sayer, A Smirnov, SR Suda (Petters), TD Toth, DR Worsnop, A Wozniak, and SR Zorn, Production mechanisms, number concentration, size distribution, chemical composition, and optical properties of sea spray aerosols, Atmos. Sci. Lett. 14(4), 207-213, doi 10.1002/asl2.441, 2013. [pdf]
[1] SR Suda (Petters), MD Petters, A Matsunaga, RC Sullivan, PJ Ziemann, and SM Kreidenweis, Hygroscopicity frequency distributions of secondary organic aerosols, J. Geophys. Res. 117, D04207, doi 10.1029/2011JD016823, 2012.* [pdf]
*This work was highlighted in Eos, the news magazine of the American Geophysical Union: E Balcerak, Efficiency of organic aerosols as cloud condensation nuclei, Eos Trans. AGU, 93(14), 150, 2012.
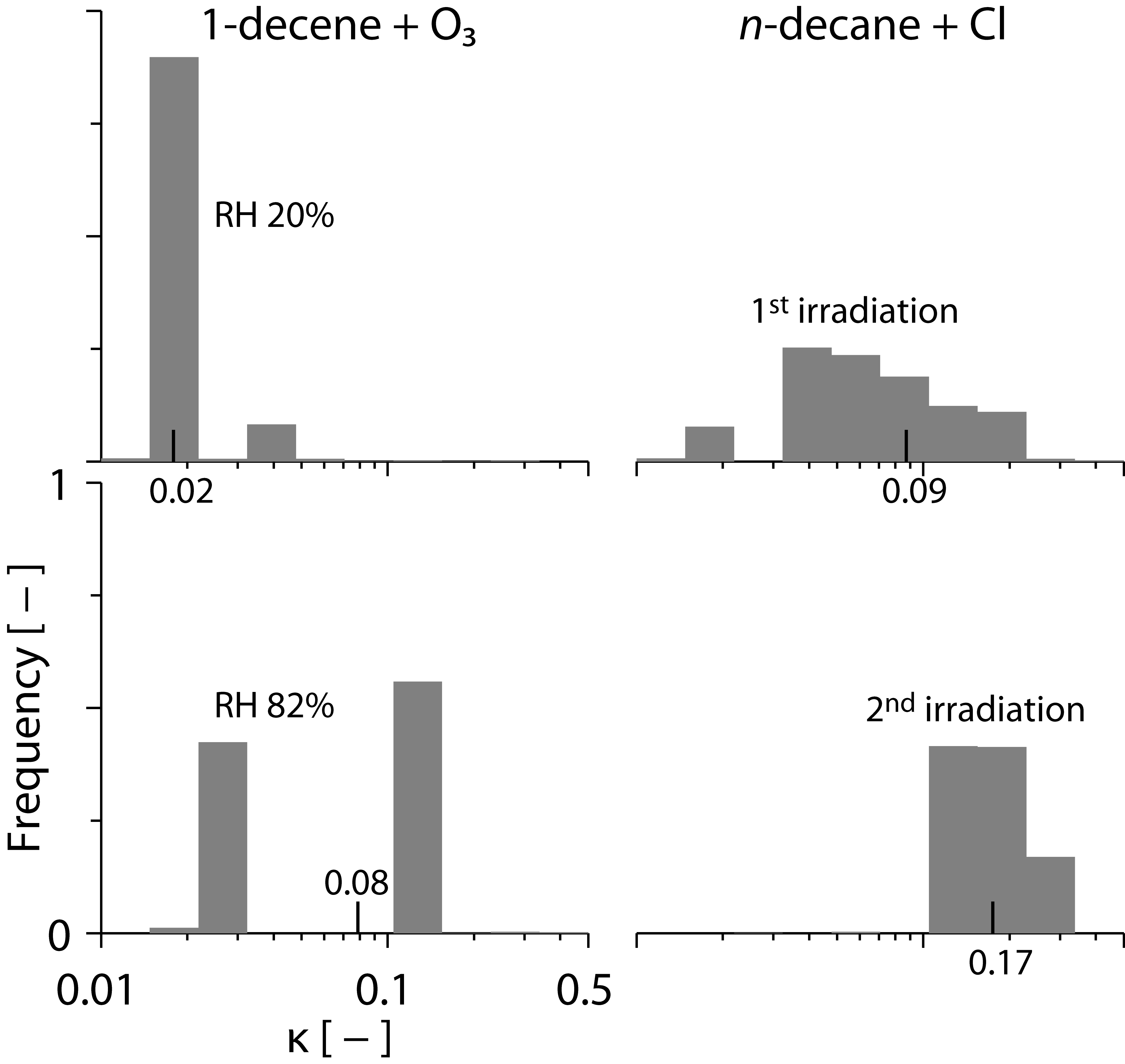
|
The hygroscopicity of different compounds found in secondary organic aerosol can shift as a result of different environmental processes (different RH during gas-phase chemistry, left panels; different amount of irradiation, right panels). We used liquid chromatography coupled to a measurement of cloud condensation nuclei (CCN) efficiency to detect these shifts and learn something about the underlying mechanism.[1]
|
Dissertation and Thesis
SS Petters (2015), On the physicochemical processes controlling organic aerosol hygroscopicity, Ph. D. Dissertation, 173 pp., North Carolina State University, Raleigh, North Carolina, 19 October. [link]
SR Suda (Petters) (2011), Hygroscopicity frequency distributions of secondary organic aerosols, M. S. Thesis, 152 pp., North Carolina State University, Raleigh, North Carolina, 23 September.** [link]
**This thesis won the Conference of Southern Graduate Schools Master Thesis Award in Math, Physical Sciences and Engineering, 2013. [link]












